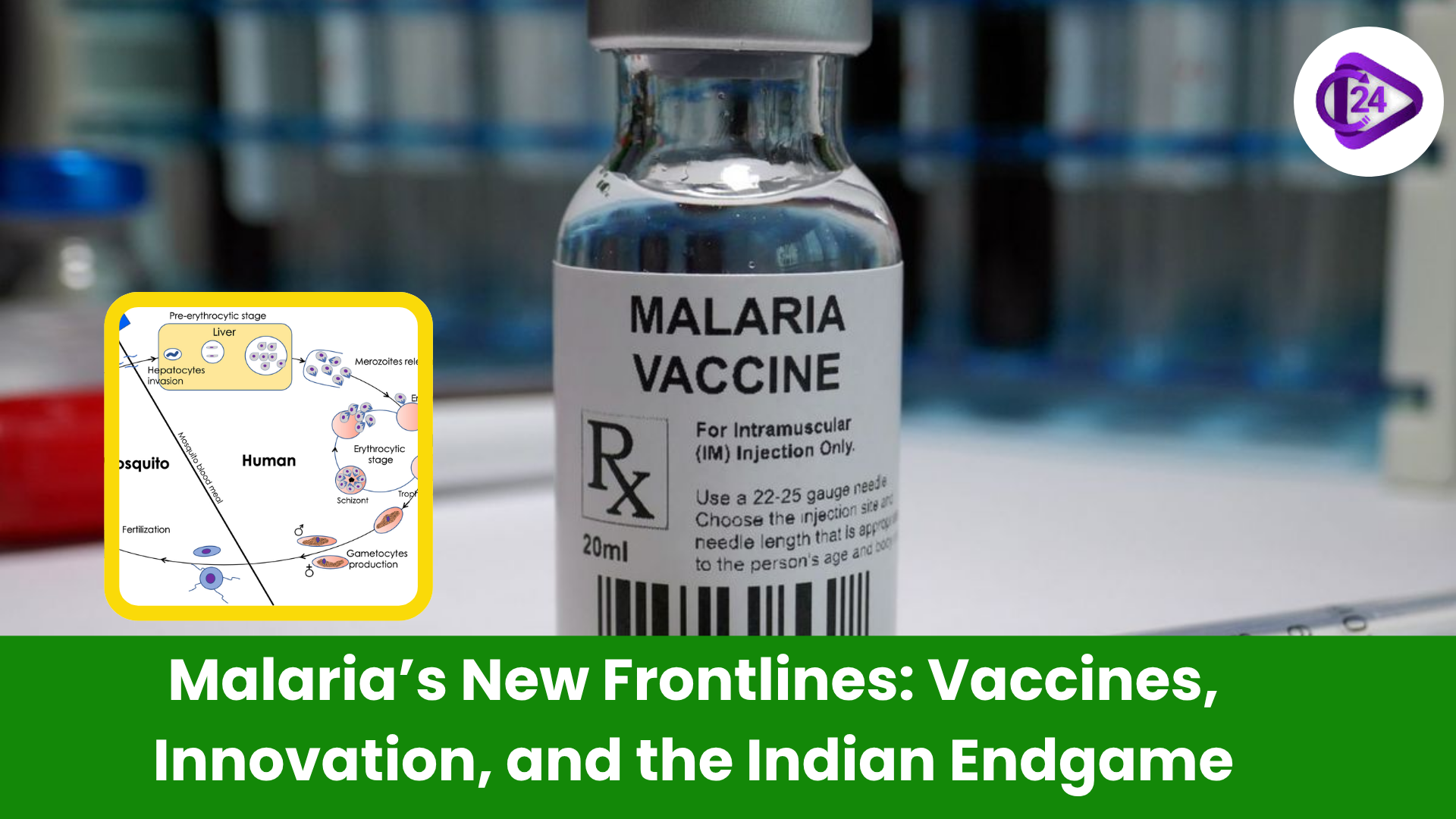
Malaria is the most ancient human plague, which is still a topical problem of public health in a great number of regions all over the world. In spite of the significant progress on a global scale, it is estimated that almost 294 million individuals were infected in 2023, with 600,000 losses of lives. India which has achieved more than 80 percent reduction in malaria burden between 2015 and 2023 are now faced with a more complex task, eliminating malaria by 2030 in the face of not just hidden reservoirs, drug-resistance but also of negligent species such as Plasmodium vivax.
The endgame against malaria is no longer in terms of evenly distributed disease control; it is precision targeting, sophisticated science and socio-political alignment. The way ahead is brightened by innovative vaccine research, local innovation, emerging biological technologies and technologies will require everlasting dedication.
Malaria in India
-
The fact that India has managed to reduce malaria is clear, but the disparities are severe in the regions. For example:
-
In 2023, there were 56 per 1,000 of the population in Lawngtlai (Mizoram).
-
More than 22 cases per 1000 were reported in Narayanpur (Chhattisgarh).
-
-
This is because the high-burden zones are mainly tribal and isolated, with low representation of healthcare facilities and diagnostic and surveillance systems.
-
India must struggle against Plasmodium falciparum and the relapse-prone Plasmodium vivax which is more difficult to find and get rid of because of the inactive stages in the liver.
Approved Vaccines
-
RTS,S/AS01 (Mosquirix):
-
Approved 2021; ~55% in year 1.
-
Has to be taken four times; it diminishes with time.
-
-
R21/ Matrix-M (Oxford-Serum Institute):
-
WHO- approved in 2023.
-
Appears with an efficacy of up to 77 per cent; only a few doses and cheaper to administer would suit Indian circumstances.
-
Whole-Parasite Vaccines
-
PfSPZ, and PfSPZ-LARC2 (Sanaria):
-
Take radiation-attenuated P. falciparum sporozoites.
-
Single-shot potential; initial testing indicates efficacy of up to 79% in early testing.
-
Can be applicable to outbreak areas or mobile populations.
Vaccine Candidates at the blood stage
PfRH5-based vaccines:
-
Homes on vital red blood cell invasion protein.
-
Provides cross-strain immunity - one of the main advantages in the ecology of parasites in India.
-
Population-level innovations: Go after Transmission
Targeting Transmission: Population-Level Innovations
Transmission-Blocking Vaccines (TBVs)
-
Pfs230D1 (Mali Trial): 78% reduction in mosquito transmission.
-
Indian Innovation -AdFalciVax:
-
ICMR-RMRC (2025) developed the first two-stage TBV in India.
-
Pre-treatment and transmission-blocking antigens are combined.
-
Stays in room condition during 9 months in the room - crucial in approaching rural outreach.
-
It is now at preclinical stages; the future challenge is human trials.
-
-
Pvs230D1M (Thailand trial):
-
Acts on P. vivax-cut off the mosquito transmission by 96%.
-
Next-Gen Platforms: mRNA and Engineered Immunity
-
mRNA-based vaccines:
-
Vaccine: Pfs25 mRNA blocks total transmission in mice, NIH-CureVac.
-
The mRNA candidate of BioNTech was put under clinical hold, which led to the problem of translation.
-
-
Antibody Engineering:
-
D1D2.v-IgG. antibody blocks RIFIN /LILRB1 association on the one hand normalizes the attack by the immune system.
-
In the pre-LAB phase, it has the potential to make vaccines more effective or develop new drugs.
-
-
Antigen Optimization:
-
Fusion of PfCSP with immune attracting molecules such as MIP3 enhances T-cell as well as antibody response.
-
Gene Drives and Genetic Editing: A Vector-Level Revolution
-
CRISPR based gene drives:
-
In the laboratory, colonies of Anopheles gambiae were eradicated by fertility-interfering genes.
-
Ecological and ethical concern; it cannot be reversed.
-
-
Mosquito Immuno-editing:
-
Genomic editing of the FREP1 gene prevents parasite development - spreads fast with gene drives.
-
-
Self-limiting vectors:
-
Infected modified mosquitoes die out sooner in a positive reinforcement creating a blockage of the spreading process.
-
Challenges in India
-
Vivax Neglect:
-
No licensed vaccine in existence; P. vivax research is grossly underfunded.
-
Primate models, such as P. cynomolgi are not readily available, and this impedes research.
-
-
Regulatory and Infrastructure Bottlenecks:
-
Lab to trials (e.g. AdFalciVax) requires industrial collaboration, immune biomarkers and GMP-quality materials.
-
The process of approval may take up to 8 years.
-
-
Systemic Gaps:
-
Scientific innovations must be accompanied by trained staff, rural diagnostics, resistance monitoring, and tactics of controlling the vectors.
-
The Road to the Future: Strategic Plan in Public Health
-
Policy and Funding:
-
India needs a “COVID-era” kind of boost — emergency money, super-speedy green-lights, and political will.
-
-
Multilateral Collaboration:
-
Exchange information with ICMR, DBT, academia, biotech firms and WHO to have international benchmarking.
-
-
Bear on Hotspot Elimination:
-
Strategies to include targeting tribal and forested areas with vaccine and control strategies.
-
-
Integration of Public Health:
-
Integrate vaccines and surveillance with community engagement and strengthening of the health systems.
-
Conclusion
Now when it comes to the malaria story of India, it is a story of not so symmetrical battlegrounds, whether on the urban outskirts and the forest depths or P. falciparum or P. vivax. India is now better prepared than ever to face the problem with powerful new vaccine platforms and homegrown solutions, such as AdFalciVax, as well as genetically targeted vector controls.



 World’s 1st Functioning AI-designed Viral Genome
World’s 1st Functioning AI-designed Viral Genome AI-led Efficiencies Could Contribute to 8% GDP Growth Target: NITI Aayog
AI-led Efficiencies Could Contribute to 8% GDP Growth Target: NITI Aayog India Projects Five-fold Growth in Space Economy to $44 Billion by 2033
India Projects Five-fold Growth in Space Economy to $44 Billion by 2033 Ministry of Tribal Affairs to Launch the Beta Version of “Adi Vaani”
Ministry of Tribal Affairs to Launch the Beta Version of “Adi Vaani” UNGA Launches Two New Initiatives to Strengthen Global Cooperation on AI Governance
UNGA Launches Two New Initiatives to Strengthen Global Cooperation on AI Governance ISRO holds Air Drop Test for Gaganyaan Mission
ISRO holds Air Drop Test for Gaganyaan Mission Tiny Gold Particles can help early detection of Parkinson’s Disease
Tiny Gold Particles can help early detection of Parkinson’s Disease National Space Day 2025 Celebrated Across India
National Space Day 2025 Celebrated Across India India-NASA Earth Observation Partnership: NISAR Satellite Launch
India-NASA Earth Observation Partnership: NISAR Satellite Launch






