
In a recent study, directed by Professor Marya Lieberman of the University of Notre Dame, various chemotherapy drugs manufactured in India by various organizations, have indeed been found to have not endured quality tests and thus, patients across more than 100 countries and afflicted with cancer are quite vulnerable. The testing in 189 samples of drugs on cancer, showed the result of about a fifth of the drugs being out of control in terms of quality standards, with Venus Remedies testing the worst. These drugs that are of the essence in treating diseases such as breast cancer, ovary cancer and leukaemia have proved to be either ineffective or terribly overdosed posing very serious side effects that could be life threatening. This has provoked issues regarding the worldwide regulation of the generic pharmaceutical sector and the safety of the health of the population.
Context
-
Research published that the chemotherapy drugs produced by Indian pharmaceutical organizations such as Venus Remedies, Zuvius Lifesciences and GLS Pharma did not pass the quality test thus putting global cancer patients at harm.
-
Such results demonstrate severe flaws in the international drug security system and authorities control.
Key Points
Study Findings
-
The survey was carried out by University of Notre Dame
-
Almost one in every five chemotherapy medication manufactured by Indian firms failed to pass quality examinations
-
Worst performer - all 8 cycles;phosphamide samples tested by Venus Remedies failed
-
Zuvius Lifesciences, GLS Pharma are other companies involved.
-
In more than 40 countries faulty drugs were distributed
Influence on Treatment of Cancer
-
Medications contained either excessively high or insufficient levels of active component
-
Not enough: Powerless treatment
-
Too much: Toxic side effects, body parts damage, and even deaths
-
Nepal, Ethiopia, Saudi Arabia and the U.S are some of the affected countries.
Drug Regulation Lapses in the globe
-
Illuminates ineffective cross border regulatory of quality of generic drugs
-
Nepal: No capacity to do cancer drug testing; did not recall a single drug
-
Brings the regulatory enforcing in India into question as the biggest supplier of generic drugs to the world
International Public Health Implications
-
In low income nations, a patient who has cancer is not able to spend money on other drugs when first medication fails
-
Reveals weakness of dependence on inexpensive generic cancer medicines all over the world
-
Raises demands of more robust international oversight and national change in quality control of drugs
Conclusion
The inefficiency of chemotherapy drugs manufactured in India raises severe concerns about drug quality and drug regulatory control in the world but exposes vulnerable patients with cancer to the risk of failure to cure this ailment. Although the role that India plays in the production of generic drugs is vital in ensuring that the rest of the world has access to medicine, control mechanisms should be intensified so as to certify the safety and efficiency of these medicines. Given the seriousness of some of the cases covered in the study, there is an extreme necessity to have a bigger regulation and transparency at a global level in the pharmaceutical industry to safeguard patients all over the world.



 World Soil Day 2025: Celebrating “Healthy Soils for Healthy Cities”
World Soil Day 2025: Celebrating “Healthy Soils for Healthy Cities”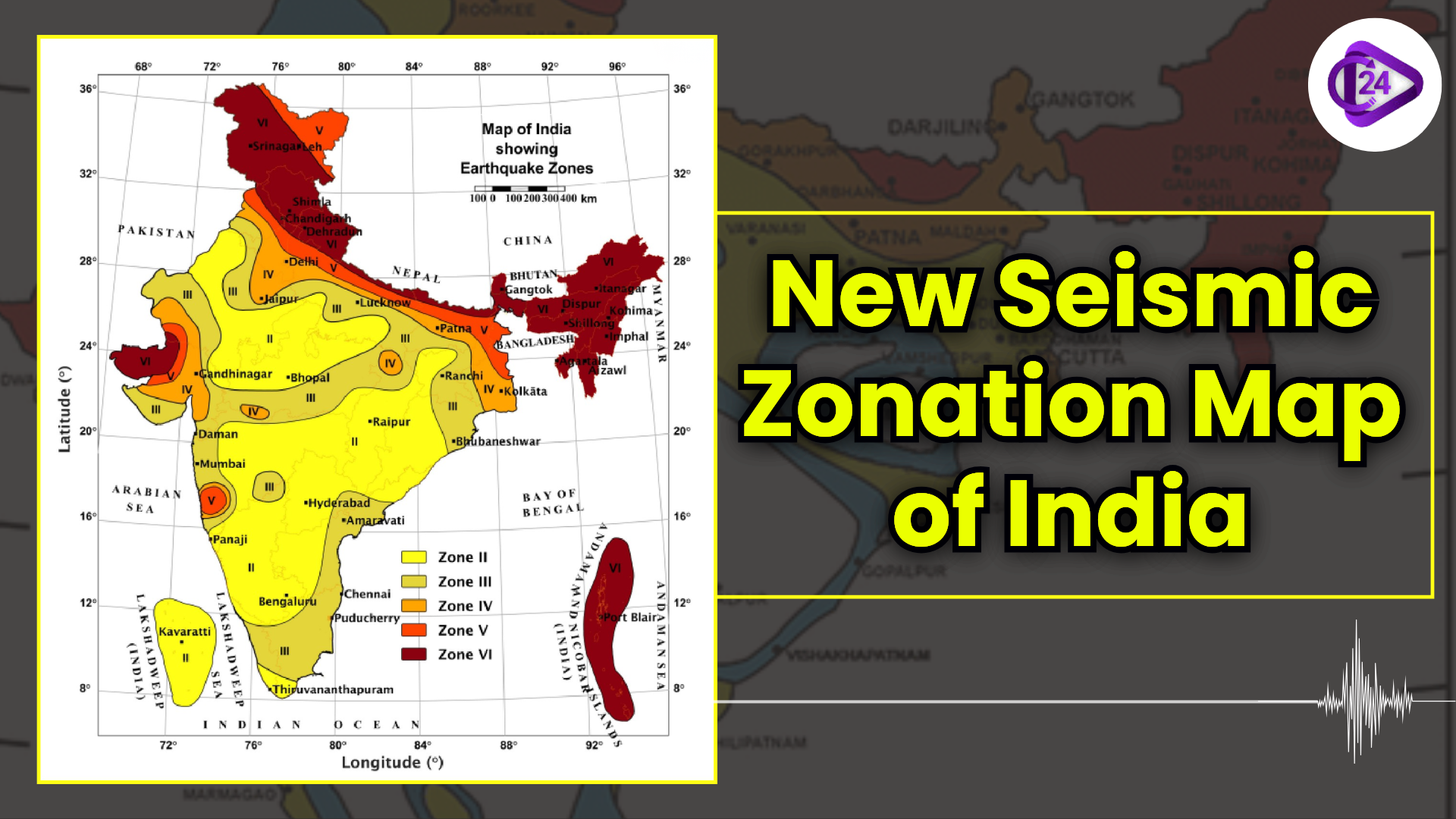 New Seismic Zonation Map of India
New Seismic Zonation Map of India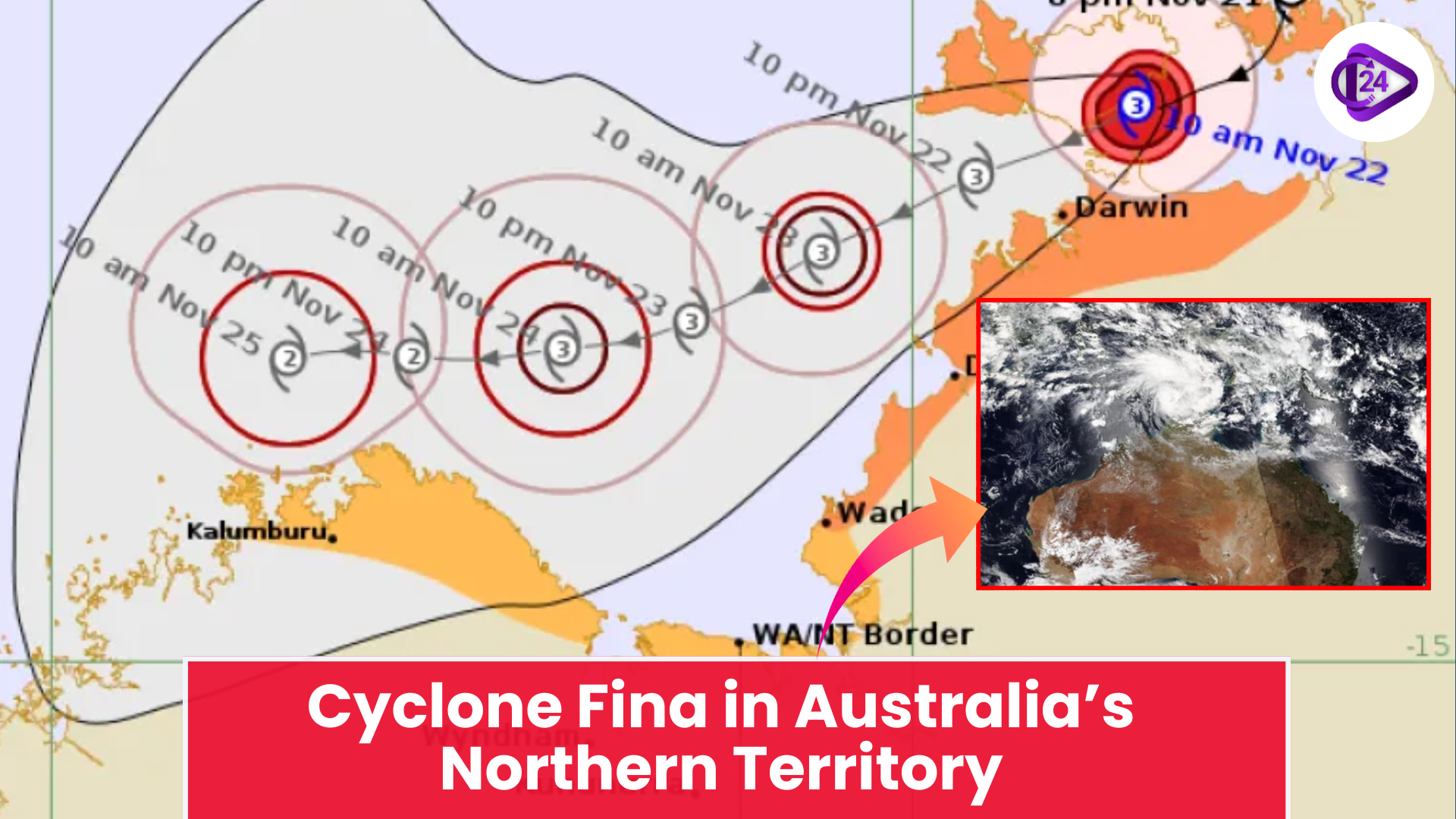 Cyclone Fina Hits Northern Australia With Destructive Force
Cyclone Fina Hits Northern Australia With Destructive Force Tiger Returns to Gujarat After 32 Years | Historic Wildlife Comeback 2025
Tiger Returns to Gujarat After 32 Years | Historic Wildlife Comeback 2025 Namdapha Butterfly Festival Showcases the Wild Heart of Arunachal Pradesh
Namdapha Butterfly Festival Showcases the Wild Heart of Arunachal Pradesh Gogabeel Lake Achieves Ramsar Status for Biodiversity and Conservation
Gogabeel Lake Achieves Ramsar Status for Biodiversity and Conservation Cyclone Montha Makes Landfall Near Kakinada, Bringing Destruction to Andhra and Odisha
Cyclone Montha Makes Landfall Near Kakinada, Bringing Destruction to Andhra and Odisha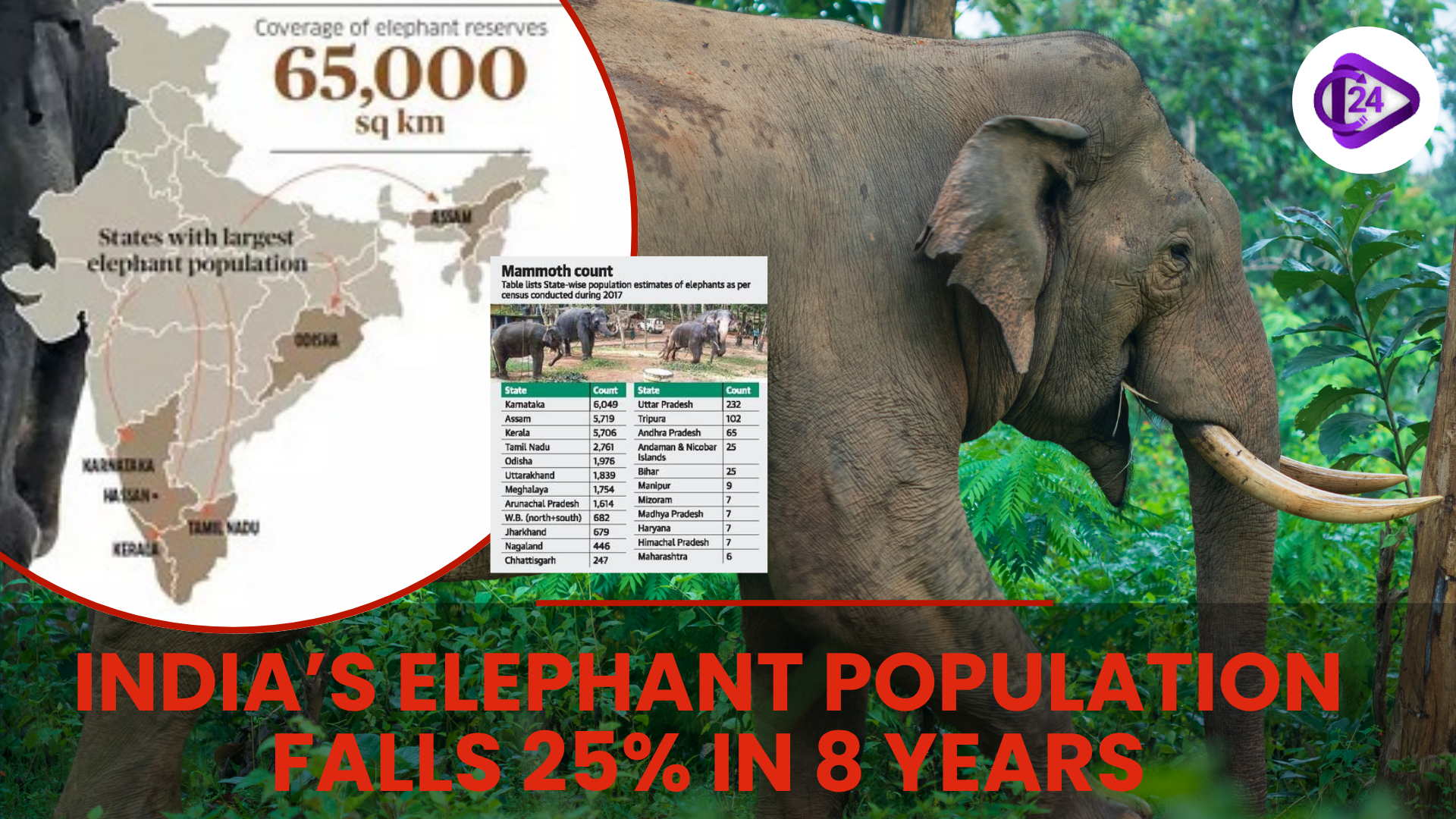 India Conducts First-Ever DNA-Based Elephant Census, Reveals Population Decline by 25%
India Conducts First-Ever DNA-Based Elephant Census, Reveals Population Decline by 25% Maharashtra Gets India’s First Cooperative CBG Plant | 12 Tonnes Biogas Daily Production
Maharashtra Gets India’s First Cooperative CBG Plant | 12 Tonnes Biogas Daily Production Pulicat Fishermen Demand Long-Term Solution as Silt Threatens Lake and Livelihoods
Pulicat Fishermen Demand Long-Term Solution as Silt Threatens Lake and Livelihoods






